IL1Interleukin-1 homologues |
|---|
| SMART accession number: | SM00125 |
|---|---|
| Description: | Cytokines with various biological functions. Interluekin 1 alpha and beta are also known as hematopoietin and catabolin. |
| Family alignment: |
There are 2006 IL1 domains in 2001 proteins in SMART's nrdb database.
Click on the following links for more information.
- Evolution (species in which this domain is found)
-
Taxonomic distribution of proteins containing IL1 domain.
This tree includes only several representative species. The complete taxonomic breakdown of all proteins with IL1 domain is also avaliable.
Click on the protein counts, or double click on taxonomic names to display all proteins containing IL1 domain in the selected taxonomic class.
- Literature (relevant references for this domain)
-
Primary literature is listed below; Automatically-derived, secondary literature is also avaliable.
- Dinarello CA
- Interleukin-1.
- Cytokine Growth Factor Rev. 1997; 8: 253-65
- Display abstract
Interleukin-1 (IL-1) is the prototypic pro-inflammatory cytokine. There are two forms of IL-1, IL-1alpha and IL-1beta and in most studies, their biological activities are indistinguishable. IL-1 affects nearly every cell type, often in concert with another pro-inflammatory cytokine, tumor necrosis factor (TNF). Although IL-1 can upregulate host defenses and function as an immunoadjuvant, IL-1 is a highly inflammatory cytokine. The margin between clinical benefit and unacceptable toxicity in humans is exceedingly narrow. In contrast, agents that reduce the production and/or activity of IL-1 are likely to have an impact on clinical medicine. The synthesis, processing, secretion and activity of IL-1, particularly IL-1beta, are tightly regulated events. A unique aspect of cytokine biology is the naturally occurring IL-1 receptor antagonist (IL-1Ra). IL-1Ra is structurally similar to IL-1beta but lacking agonist activity is used in clinical trials to reduce disease severity. In addition, regulation of IL-1 activity extends to low numbers of surface receptors, circulating soluble receptors and a cell surface "decoy" receptor to down-regulate responses to IL-1beta. This review updates the current knowledge on IL-1.
- Patarca R, Fletcher MA
- Interleukin-1: basic science and clinical applications.
- Crit Rev Oncog. 1997; 8: 143-88
- Kurzrock R, Wetzler M, Estrov Z, Talpaz M
- Interleukin-1 and its inhibitors: a biologic and therapeutic model for the role of growth regulatory factors in leukemias.
- Cytokines Mol Ther. 1995; 1: 177-84
- Display abstract
Production of growth factors may provide a mechanism for disease evolution in some leukemias. Interleukin-1 is a plelotropic cytokine with the ability to synergize with other growth factors as well as to stimulate their production and release. Autocrine and/or paracrine secretion of interleukin-1 has been implicated in the pathogenesis of both chronic and acute myelogenous leukemia. Recently, a series of both specific and nonspecific IL-1 inhibitory molecules have been identified. These include IL-1 receptor antagonist, soluble IL-1 receptors, IL-1-converting enzyme inhibitor, IL-4, IL-10 and IL-1-antisense. Early experiments demonstrating the ability of some of these molecules to inhibit acute and chronic myelogenous leukemia growth suggest that clinical trials of these compounds may provide a novel management approach in these malignancies. Here we review the potential biologic and therapeutic role of IL-1 and its inhibitors in the myeloid leukemias.
- Metabolism (metabolic pathways involving proteins which contain this domain)
-
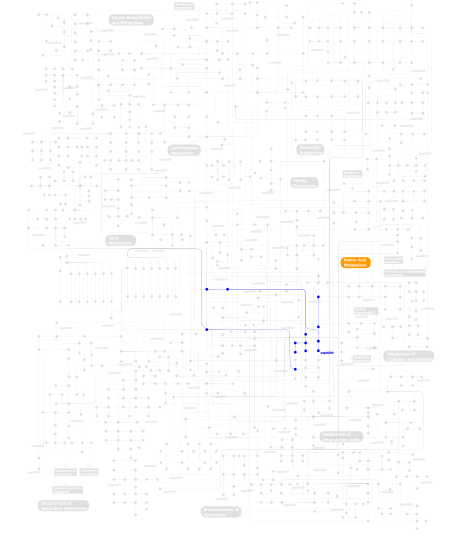
Click the image to view the interactive version of the map in iPath% proteins involved KEGG pathway ID Description 19.19 map04060 Cytokine-cytokine receptor interaction 15.15 map04640 Hematopoietic cell lineage 15.15 map04010 MAPK signaling pathway 15.15 map04210 Apoptosis 15.15 map04940 Type I diabetes mellitus 9.09 map04620 Toll-like receptor signaling pathway 9.09 map05010 Alzheimer's disease 1.01 map00970 Aminoacyl-tRNA biosynthesis 1.01  map00290
map00290Valine, leucine and isoleucine biosynthesis This information is based on mapping of SMART genomic protein database to KEGG orthologous groups. Percentage points are related to the number of proteins with IL1 domain which could be assigned to a KEGG orthologous group, and not all proteins containing IL1 domain. Please note that proteins can be included in multiple pathways, ie. the numbers above will not always add up to 100%.
- Structure (3D structures containing this domain)
3D Structures of IL1 domains in PDB
PDB code Main view Title 1hib 
THE STRUCTURE OF AN INTERLEUKIN-1 BETA MUTANT WITH REDUCED BIOACTIVITY SHOWS MULTIPLE SUBTLE CHANGES IN CONFORMATION THAT AFFECT PROTEIN-PROTEIN RECOGNITION 1i1b 
CRYSTAL STRUCTURE OF RECOMBINANT HUMAN INTERLEUKIN-1BETA AT 2.0 ANGSTROMS RESOLUTION 1ilr 
CRYSTAL STRUCTURE OF THE INTERLEUKIN-1 RECEPTOR ANTAGONIST 1ilt 
X-RAY STRUCTURE OF INTERLEUKIN-1 RECEPTOR ANTAGONIST AT 2.0 ANGSTROMS RESOLUTION 1iob 
INTERLEUKIN-1 BETA FROM JOINT X-RAY AND NMR REFINEMENT 1ira 
COMPLEX OF THE INTERLEUKIN-1 RECEPTOR WITH THE INTERLEUKIN-1 RECEPTOR ANTAGONIST (IL1RA) 1irp 
SOLUTION STRUCTURE OF HUMAN INTERLEUKIN-1 RECEPTOR ANTAGONIST PROTEIN 1itb 
TYPE-1 INTERLEUKIN-1 RECEPTOR COMPLEXED WITH INTERLEUKIN-1 BETA 1j0s 
Solution structure of the human interleukin-18 1l2h 
Crystal structure of Interleukin 1-beta F42W/W120F mutant 1md6 
High resolution crystal structure of murine IL-1F5 reveals unique loop conformation for specificity 1s0l 
Interleukin 1 beta mutant F42W 1t4q 
Interleukin 1 beta F101W 1too 
Interleukin 1B Mutant F146W 1tp0 
Triple mutation in interleukin 1 beta cavity:replacement of phenylalanines with tryptophan. 1twe 
INTERLEUKIN 1 BETA MUTANT F101Y 1twm 
Interleukin-1 Beta Mutant F146Y 21bi 
INTERLEUKIN-1 BETA (IL-1 BETA) (MUTANT WITH CYS 71 REPLACED BY ALA) (C71A) 2i1b 
CRYSTALLOGRAPHIC REFINEMENT OF INTERLEUKIN-1 BETA AT 2.0 ANGSTROMS RESOLUTION 2ila 
STRUCTURE OF INTERLEUKIN 1ALPHA AT 2.7-ANGSTROMS RESOLUTION 2irt 
INITIAL CRYSTALLOGRAPHIC ANALYSES OF A RECOMBINANT INTERLEUKIN-1 RECEPTOR ANTAGONIST PROTEIN 2kh2 
Solution structure of a scFv-IL-1B complex 2kki 
Solution structure of human Interleukin 1a 2l5x 
Solution structure of IL1A-S100A13 complex 2mib 
THE STRUCTURE OF MURINE INTERLEUKIN-1 BETA AT 2.8 ANGSTROMS RESOLUTION 2nvh 
Determination of Solvent Content in Cavities in Interleukin-1 Using Experimentally-Phased Electron Density 2vxt 
Crystal structure of human IL-18 complexed to murine reference antibody 125-2H Fab 2wry 
Crystal structure of chicken cytokine interleukin 1 beta 31bi 
INTERLEUKIN-1 BETA (IL-1 BETA) (MUTANT WITH CYS 71 REPLACED BY SER) (C71S) 3f62 
Crystal Structure of Human IL-18 in complex with Ectromelia virus IL-18 Binding Protein 3ltq 
Structure of Interleukin 1B solved by SAD using an inserted Lanthanide Binding Tag 3nj5 
Crystal structure of chicken IL-1 hydrophobic cavity mutant 157 3o4o 
Crystal structure of an Interleukin-1 receptor complex 3pok 
Interleukin-1-beta LBT L3 Mutant 3wo2 
3WO2 3wo3 
3WO3 3wo4 
3WO4 41bi 
INTERLEUKIN-1 BETA (IL-1 BETA) (MUTANT WITH CYS 8 REPLACED BY ALA (C8A) 4dep 
Structure of the IL-1b signaling complex 4g6j 
Crystal structure of human IL-1beta in complex with the therapeutic antibody binding fragment of canakinumab 4g6m 
Crystal strucure of human IL-1beta in complex with therapeutic antibody binding fragment of gevokizumab 4gaf 
Crystal structure of EBI-005, a chimera of human IL-1beta and IL-1Ra, bound to human Interleukin-1 receptor type 1 4gai 
Crystal structure of EBI-005, a chimera of human IL-1beta and IL-1Ra 4hjj 
Structure Reveals Function of the Dual Variable Domain Immunoglobulin (DVD-Ig) Molecule 4i1b 
FUNCTIONAL IMPLICATIONS OF INTERLEUKIN-1BETA BASED ON THE THREE-DIMENSIONAL STRUCTURE 4ize 
4IZE 4p0j 
4P0J 4p0k 
4P0K 4p0l 
4P0L 4r6u 
4R6U 4x37 
4X37 4x38 
4X38 4x39 
4X39 4x3a 
4X3A 4xfs 
4XFS 5bow 
5BOW 5bvp 
5BVP 5i1b 
A COMPARISON OF THE HIGH RESOLUTION STRUCTURES OF HUMAN AND MURINE INTERLEUKIN-1B 6i1b 
HIGH-RESOLUTION THREE-DIMENSIONAL STRUCTURE OF INTERLEUKIN-1 BETA IN SOLUTION BY THREE-AND FOUR-DIMENSIONAL NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY 7i1b 
HIGH-RESOLUTION THREE-DIMENSIONAL STRUCTURE OF INTERLEUKIN-1 BETA IN SOLUTION BY THREE-AND FOUR-DIMENSIONAL NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY 8i1b 
A COMPARISON OF THE HIGH RESOLUTION STRUCTURES OF HUMAN AND MURINE INTERLEUKIN-1B 9ilb 
HUMAN INTERLEUKIN-1 BETA - Links (links to other resources describing this domain)
-
PROSITE IL1_DOMAIN

