P4HcProlyl 4-hydroxylase alpha subunit homologues. |
|---|
| SMART accession number: | SM00702 |
|---|---|
| Description: | Mammalian enzymes catalyse hydroxylation of collagen, for example. Prokaryotic enzymes might catalyse hydroxylation of antibiotic peptides. These are 2-oxoglutarate-dependent dioxygenases, requiring 2-oxoglutarate and dioxygen as cosubstrates and ferrous iron as a cofactor. |
| Interpro abstract (IPR006620): | Mammalian prolyl 4-hydroxylase alpha catalyses the posttranslational formation of 4- hydroxyproline in -xaa-pro-gly-sequences in collagens and other proteins. Prokaryotic enzymes might catalyse hydroxylation of antibiotic peptides. These are 2-oxoglutarate-dependent dioxygenases, requiring 2-oxoglutarate and dioxygen as cosubstrates and ferrous iron as a cofactor [ (PUBMED:11276424) ]. |
| GO process: | oxidation-reduction process (GO:0055114) |
| GO function: | oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen (GO:0016705), iron ion binding (GO:0005506), L-ascorbic acid binding (GO:0031418) |
| Family alignment: |
There are 28654 P4Hc domains in 28559 proteins in SMART's nrdb database.
Click on the following links for more information.
- Evolution (species in which this domain is found)
-
Taxonomic distribution of proteins containing P4Hc domain.
This tree includes only several representative species. The complete taxonomic breakdown of all proteins with P4Hc domain is also avaliable.
Click on the protein counts, or double click on taxonomic names to display all proteins containing P4Hc domain in the selected taxonomic class.
- Cellular role (predicted cellular role)
-
Binding / catalysis: Proline hydroxylation
- Literature (relevant references for this domain)
-
Primary literature is listed below; Automatically-derived, secondary literature is also avaliable.
- Aravind L, Koonin EV
- The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases.
- Genome Biol. 2001; 2: 7-7
- Display abstract
BACKGROUND: Protein fold recognition using sequence profile searches frequently allows prediction of the structure and biochemical mechanisms of proteins with an important biological function but unknown biochemical activity. Here we describe such predictions resulting from an analysis of the 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenases, a class of enzymes that are widespread in eukaryotes and bacteria and catalyze a variety of reactions typically involving the oxidation of an organic substrate using a dioxygen molecule. RESULTS: We employ sequence profile analysis to show that the DNA repair protein AlkB, the extracellular matrix protein leprecan, the disease-resistance-related protein EGL-9 and several uncharacterized proteins define novel families of enzymes of the 2OG-Fe(II) oxygenase superfamily. The identification of AlkB as a member of the 2OG-Fe(II) oxygenase superfamily suggests that this protein catalyzes oxidative detoxification of alkylated bases. More distant homologs of AlkB were detected in eukaryotes and in plant RNA viruses, leading to the hypothesis that these proteins might be involved in RNA demethylation. The EGL-9 protein from Caenorhabditis elegans is necessary for normal muscle function and its inactivation results in resistance against paralysis induced by the Pseudomonas aeruginosa toxin. EGL-9 and leprecan are predicted to be novel protein hydroxylases that might be involved in the generation of substrates for protein glycosylation. CONCLUSIONS: Here, using sequence profile searches, we show that several previously undetected protein families contain 2OG-Fe(II) oxygenase fold. This allows us to predict the catalytic activity for a wide range of biologically important, but biochemically uncharacterized proteins from eukaryotes and bacteria.
- Aravind L, Koonin EV
- The DNA-repair protein AlkB, EGL-9, and leprecan define new families of2-oxoglutarate- and iron-dependent dioxygenases.
- Genome Biol. 2001; 2: 7-7
- Display abstract
BACKGROUND: Protein fold recognition using sequence profile searchesfrequently allows prediction of the structure and biochemical mechanismsof proteins with an important biological function but unknown biochemicalactivity. Here we describe such predictions resulting from an analysis ofthe 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenases, a class ofenzymes that are widespread in eukaryotes and bacteria and catalyze avariety of reactions typically involving the oxidation of an organicsubstrate using a dioxygen molecule. RESULTS: We employ sequence profileanalysis to show that the DNA repair protein AlkB, the extracellularmatrix protein leprecan, the disease-resistance-related protein EGL-9 andseveral uncharacterized proteins define novel families of enzymes of the2OG-Fe(II) oxygenase superfamily. The identification of AlkB as a memberof the 2OG-Fe(II) oxygenase superfamily suggests that this proteincatalyzes oxidative detoxification of alkylated bases. More distanthomologs of AlkB were detected in eukaryotes and in plant RNA viruses,leading to the hypothesis that these proteins might be involved in RNAdemethylation. The EGL-9 protein from Caenorhabditis elegans is necessaryfor normal muscle function and its inactivation results in resistanceagainst paralysis induced by the Pseudomonas aeruginosa toxin. EGL-9 andleprecan are predicted to be novel protein hydroxylases that might beinvolved in the generation of substrates for protein glycosylation.CONCLUSIONS: Here, using sequence profile searches, we show that severalpreviously undetected protein families contain 2OG-Fe(II) oxygenase fold.This allows us to predict the catalytic activity for a wide range ofbiologically important, but biochemically uncharacterized proteins fromeukaryotes and bacteria.
- Friedman L, Higgin JJ, Moulder G, Barstead R, Raines RT, Kimble J
- Prolyl 4-hydroxylase is required for viability and morphogenesis inCaenorhabditis elegans.
- Proc Natl Acad Sci U S A. 2000; 97: 4736-41
- Display abstract
The genome of Caenorhabditis elegans possesses two genes, dpy-18 andphy-2, that encode alpha subunits of the enzyme prolyl 4-hydroxylase. Wehave generated deletions within each gene to eliminate prolyl4-hydroxylase activity from the animal. The dpy-18 mutant has an aberrantbody morphology, consistent with a role of prolyl 4-hydroxylase information of the body cuticle. The phy-2 mutant is phenotypically wildtype. However, the dpy-18; phy-2 double mutant is not viable, suggestingan essential role for prolyl 4-hydroxylase that is normally accomplishedby either dpy-18 or phy-2. The effects of the double mutation weremimicked by small-molecule inhibitors of prolyl 4-hydroxylase, validatingthe genetic results and suggesting that C. elegans can serve as a modelsystem for the discovery of new inhibitors.
- Kivirikko KI, Myllyla R, Pihlajaniemi T
- Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with fourcosubstrates and a multifunctional subunit.
- FASEB J. 1989; 3: 1609-17
- Display abstract
Prolyl 4-hydroxylase (EC 1.14.11.2) catalyzes the formation of4-hydroxyproline in collagens by the hydroxylation of proline residues inX-Pro-Gly sequences. The reaction requires Fe2+, 2-oxoglutarate, O2, andascorbate and involves an oxidative decarboxylation of 2-oxoglutarate.Ascorbate is not consumed during most catalytic cycles, but the enzymealso catalyzes decarboxylation of 2-oxoglutarate without subsequenthydroxylation, and ascorbate is required as a specific alternative oxygenacceptor in such uncoupled reaction cycles. A number of compounds inhibitprolyl 4-hydroxylase competitively with respect to some of itscosubstrates or the peptide substrate, and recently many suicideinactivators have also been described. Such inhibitors and inactivatorsare of considerable interest, because the prolyl 4-hydroxylase reactionwould seem a particularly suitable target for chemical regulation of theexcessive collagen formation found in patients with various fibroticdiseases. The active prolyl 4-hydroxylase is an alpha 2 beta 2 tetramer,consisting of two different types of inactive monomer and probablycontaining two catalytic sites per tetramer. The large catalytic site maybe cooperatively built up of both the alpha and beta subunits, but thealpha subunit appears to contribute the major part. The beta subunit hasbeen found to be identical to the enzyme protein disulfide isomerase and amajor cellular thyroid hormone-binding protein and shows partial homologywith a phosphoinositide-specific phospholipase C, thioredoxins, and theestrogen-binding domain of the estrogen receptor. The COOH-terminus ofthis beta subunit has the amino acid sequence Lys-Asp-Glu-Leu, which wasrecently suggested to be necessary for the retention of a polypeptidewithin the lumen of the endoplasmic reticulum. The alpha subunit does nothave this COOH-terminal sequence, and thus one function of the betasubunit in the prolyl 4-hydroxylase tetramer appears to be to retain theenzyme within this cell organelle.
- Chen-Kiang S, Cardinale GJ, Udenfriend S
- Homology between a prolyl hydroxylase subunit and a tissue protein thatcrossreacts immunologically with the enzyme.
- Proc Natl Acad Sci U S A. 1977; 74: 4420-4
- Display abstract
A protein, enzymatically inactive but immunologically related to prolylhydroxylase (prolyl-glycyl-peptide, 2-oxoglutarate:oxygen oxidoreductase;EC 1.14.11.2) (cross-reacting protein), has been purified to nearhomogeneity from skin of newborn rats. The purified protein has amolecular weight of 60,000 on gel filtration and sodium dodecyl sulfategel electrophoresis, corresponding to that of the smaller of the twodissimilar subunits of the enzyme. The two subunits of prolyl hydroxylasediffer markedly from one another in their amino acid compositions, butcrossreating protein and the smaller subunit are very similar incomposition. On antibody-affinity chromatography both subunits reactedwith the antibody developed against the intact enzyme. Neithercrossreacting protein nor the 60,000 molecular weight subunit was adsorbedonto concanavalin A, which adsorbed the intact enzyme as well as thelarger subunit. It would appear that crossreacting protein is identical toone of the subunits of prolyl hydroxylase or metabolically related to it.
- Metabolism (metabolic pathways involving proteins which contain this domain)
-
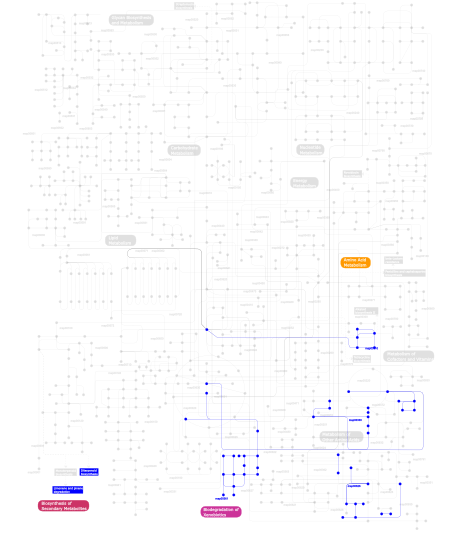
Click the image to view the interactive version of the map in iPath% proteins involved KEGG pathway ID Description 48.31  map00330
map00330Arginine and proline metabolism 24.72  map00310
map00310Lysine degradation 21.35 map05211 Renal cell carcinoma 2.25 map00904 Diterpenoid biosynthesis 1.12  map00361
map00361gamma-Hexachlorocyclohexane degradation 1.12  map00626
map00626Naphthalene and anthracene degradation 1.12 map00903 Limonene and pinene degradation This information is based on mapping of SMART genomic protein database to KEGG orthologous groups. Percentage points are related to the number of proteins with P4Hc domain which could be assigned to a KEGG orthologous group, and not all proteins containing P4Hc domain. Please note that proteins can be included in multiple pathways, ie. the numbers above will not always add up to 100%.
- Structure (3D structures containing this domain)
3D Structures of P4Hc domains in PDB
PDB code Main view Title 2g19 
Cellular Oxygen Sensing: Crystal Structure of Hypoxia-Inducible Factor Prolyl Hydroxylase (PHD2) 2g1m 
Cellular Oxygen Sensing: Crystal Structure of Hypoxia-Inducible Factor Prolyl Hydroxylase (PHD2) 2hbt 
Crystal structure of HIF prolyl hydroxylase EGLN-1 in complex with a biologically active inhibitor 2hbu 
Crystal structure of HIF prolyl hydroxylase EGLN-1 in complex with a biologically active inhibitor 2jig 
Crystal structure of Chlamydomonas reinhrdtii prolyl-4 hydroxylase type I complexed with zinc and pyridine-2,4-dicarboxylate 2jij 
Crystal structure of the apo form of Chlamydomonas reinhardtii prolyl- 4 hydroxylase type I 2v4a 
Crystal structure of the SeMet-labeled prolyl-4 hydroxylase (P4H) type I from green algae Chlamydomonas reinhardtii. 2y33 
S-nitrosylated PHD2 (GSNO soaked) in complex with Zn(II) and UN9 2y34 
S-nitrosylated PHD2 (NO exposed) in complex with Fe(II) and UN9 3dkq 
Crystal structure of Putative Oxygenase (YP_001051978.1) from SHEWANELLA BALTICA OS155 at 2.26 A resolution 3gze 
Algal prolyl 4-hydroxylase complexed with zinc and (Ser-Pro)5 peptide substrate 3hqr 
PHD2:Mn:NOG:HIF1-alpha substrate complex 3hqu 
PHD2:Fe:UN9:partial HIF1-alpha substrate complex 3itq 
Crystal Structure of a Prolyl 4-Hydroxylase from Bacillus anthracis 3kt1 
Crystal structure of Tpa1 from Saccharomyces cerevisiae, a component of the messenger ribonucleoprotein complex 3kt4 
Crystal structure of Tpa1 from Saccharomyces cerevisiae, a component of the messenger ribonucleoprotein complex 3kt7 
Crystal structure of Tpa1 from Saccharomyces cerevisiae, a component of the messenger ribonucleoprotein complex 3mgu 
Saccharomyces cerevisiae Tpa1 3ouh 
PHD2-R127 with JNJ41536014 3oui 
PHD2-R717 with 40787422 3ouj 
PHD2 with 2-Oxoglutarate 4bqw 
HIF prolyl hydroxylase 2 (PHD2/ EGLN1) in complex with Mn(II) and 2-(4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxamido)acetic acid 4bqx 
HIF prolyl hydroxylase 2 (PHD2/ EGLN1) in complex with Mn(II) and N-[(1-chloro-4-hydroxyisoquinolin-3-yl)carbonyl]glycine (IOX3/UN9) 4bqy 
HIF prolyl hydroxylase 2 (PHD2/ EGLN1) in complex with Fe(II) and N-[(1-chloro-4-hydroxyisoquinolin-3-yl)carbonyl]alanine 4iw3 
Crystal structure of a Pseudomonas putida prolyl-4-hydroxylase (P4H) in complex with elongation factor Tu (EF-Tu) 4j25 
Crystal structure of a Pseudomonas putida prolyl-4-hydroxylase (P4H) 4jzr 
Structure of Prolyl Hydroxylase Domain-containing Protein (PHD) with Inhibitors 4kbz 
Crystal Structure of Hypoxia-Inducible Factor Prolyl Hydroxylase (PHD2) with (S)-{2-[2-(5-Cyano-3-hydroxy-pyridin-2-yl)-thiazol-4-yl]-acetylamino}-phenyl-acetic acid 4nhk 
4NHK 4nhl 
4NHL 4nhm 
4NHM 4nhx 
4NHX 4nhy 
4NHY 4uwd 
4UWD 5a3u 
5A3U 5c5t 
5C5T 5c5u 
5C5U 5hv0 
5HV0 5hv4 
5HV4 5iat 
5IAT 5iav 
5IAV 5iax 
5IAX 5l9b 
5L9B 5l9r 
5L9R 5l9v 
5L9V 5la9 
5LA9 5las 
5LAS 5lat 
5LAT 5lb6 
5LB6 5lbb 
5LBB 5lbc 
5LBC 5lbe 
5LBE 5lbf 
5LBF - Links (links to other resources describing this domain)
-
INTERPRO IPR006620

