UBQUbiquitin homologues |
|---|
| SMART accession number: | SM00213 |
|---|---|
| Description: | Ubiquitin-mediated proteolysis is involved in the regulated turnover of proteins required for controlling cell cycle progression |
| Interpro abstract (IPR000626): | Ubiquitin is a globular protein, the last four C-terminal residues (Leu-Arg-Gly-Gly) extending from the compact structure to form a 'tail', important for its function. The latter is mediated by the covalent conjugation of ubiquitin to target proteins, by an isopeptide linkage between the C-terminal glycine and the epsilon amino group of lysine residues in the target proteins. Ubiquitin is a protein of 76 amino acid residues, found in all eukaryotic cells and whose sequence is extremely well conserved from protozoan to vertebrates. Ubiquitin acts through its post-translational attachment (ubiquitinylation) to other proteins, where these modifications alter the function, location or trafficking of the protein, or targets it for destruction by the 26S proteasome [ (PUBMED:15454246) ]. Ubiquitin is expressed as three different precursors: a polymeric head-to-tail concatemer of identical units (polyubiquitin), and two N-terminal ubiquitin moieties, UbL40 and UbS27, that are fused to the ribosomal polypeptides L40 and S27, respectively. Specific endopeptidases cleave these precursor molecules [ (PUBMED:15571815) ] to release ubiquitin moieties that are identical in sequence and contribute to the ubiquitin pool [ (PUBMED:16185873) ]. Some organisms express additional ubiquitin fusion proteins [ (PUBMED:12729753) ]. Furthermore, there are several ubiquitin-like proteins derived from ubiquitin [ (PUBMED:12826404) ]. This entry represents a domain characteristic of ubiquitin (Ub) and ubiquitin-like (Ubl) proteins such as SUMO [ (PUBMED:17491593) (PUBMED:15479240) ] and Nedd8 [ (PUBMED:9857030) ]. |
| GO function: | protein binding (GO:0005515) |
| Family alignment: |
There are 49174 UBQ domains in 38515 proteins in SMART's nrdb database.
Click on the following links for more information.
- Evolution (species in which this domain is found)
-
Taxonomic distribution of proteins containing UBQ domain.
This tree includes only several representative species. The complete taxonomic breakdown of all proteins with UBQ domain is also avaliable.
Click on the protein counts, or double click on taxonomic names to display all proteins containing UBQ domain in the selected taxonomic class.
- Literature (relevant references for this domain)
-
Primary literature is listed below; Automatically-derived, secondary literature is also avaliable.
- Bedford MT, Leder P
- The FF domain: a novel motif that often accompanies WW domains.
- Trends Biochem Sci. 1999; 24: 264-5
- Hofmann K, Bucher P
- The PCI domain: a common theme in three multiprotein complexes.
- Trends Biochem Sci. 1998; 23: 204-5
- Haas AL
- Introduction: evolving roles for ubiquitin in cellular regulation.
- FASEB J. 1997; 11: 1053-4
- Haas AL, Siepmann TJ
- Pathways of ubiquitin conjugation.
- FASEB J. 1997; 11: 1257-68
- Display abstract
The covalent attachment of the polypeptide ubiquitin to proteins marks them for degradation by the ubiquitin/26S proteasome-dependent degradation pathway. This pathway functions in regulating many fundamental processes required for cell viability. Phylogenetic analysis of ubiquitin sequences reveals greater variability among lower eukaryotes and defines essential residues, many of which are conserved among the three ubiquitin-like proteins known to undergo parallel ligation pathways. The hierarchical design of the ubiquitin conjugation mechanism provides great flexibility for the divergent evolution of new functions mediated by this posttranslational modification. Within this hierarchy, a single ubiquitin-activating enzyme provides charged intermediates to multiple targeting pathways defined by cognate ubiquitin carrier protein (E2)/ligase (E3) pairs. Sequence analysis of E2 isozymes shows that the E2 superfamily is composed of distinct function-specific families. The apparent lack of E2/E3 specificity suggested in the literature results from the presence of multiple isozymes within many E2 families and erroneous family assignments based on incomplete data sets. Other apparent inconsistencies are explained by interfamily sequence relationships among some E2 isoforms.
- Hershko A
- Roles of ubiquitin-mediated proteolysis in cell cycle control.
- Curr Opin Cell Biol. 1997; 9: 788-99
- Display abstract
Selective degradation of cyclins, inhibitors of cyclin-dependent kinases and anaphase inhibitors is responsible for several major cell cycle transitions. The degradation of these cell cycle regulators is controlled by the action of ubiquitin-protein-ligase complexes, which target the regulators for degradation by the 26S proteasome. Recent results indicate that two types of multisubunit ubiquitin ligase complexes, which are connected to the protein kinase regulatory network of the cell cycle in different ways, are responsible for the specific and programmed degradation of many cell cycle regulators.
- Saitoh H, Pu RT, Dasso M
- SUMO-1: wrestling with a new ubiquitin-related modifier.
- Trends Biochem Sci. 1997; 22: 374-6
- Hochstrasser M
- Ubiquitin-dependent protein degradation.
- Annu Rev Genet. 1996; 30: 405-39
- Display abstract
A growing number of cellular regulatory mechanisms are being linked to protein modification by the polypeptide ubiquitin. These include key transitions in the cell cycle, class I antigen processing, signal transduction pathways, and receptor-mediated endocytosis. In most, but not all, of these examples, ubiquitination of a protein leads to its degradation by the 26S proteasome. Following attachment of ubiquitin to a substrate and binding of the ubiquitinated protein to the proteasome, the bound substrate must be unfolded (and eventually deubiquitinated) and translocated through a narrow set of channels that leads to the proteasome interior, where the polypeptide is cleaved into short peptides. Protein ubiquitination and deubiquitination are both mediated by large enzyme families, and the proteasome itself comprises a family of related but functionally distinct particles. This diversity underlies both the high substrate specificity of the ubiquitin system and the variety of regulatory mechanisms that it serves.
- Hofmann K, Bucher P
- The UBA domain: a sequence motif present in multiple enzyme classes of the ubiquitination pathway.
- Trends Biochem Sci. 1996; 21: 172-3
- Isaksson A, Musti AM, Bohmann D
- Ubiquitin in signal transduction and cell transformation.
- Biochim Biophys Acta. 1996; 1288: 219-219
- Display abstract
Since the discovery of ubiquitin-dependent protein degradation almost two decades ago, great strides have been made towards a detailed understanding of the biochemistry of this process (reviewed in [1-3]). It was, however, only in recent years that the physiological role of the ubiquitin system in signal transduction and the regulation of several cell functions started to be appreciated and experimentally addressed. As with other principal mechanisms of signal transduction, such as phosphorylation or GTP hydrolysis, much of the information regarding the role of the ubiquitin system as a component of cell regulation and signaling cascades, was gained in studies of transformation and the control of cell growth. It seems, however, that ubiquitin-dependent proteolysis, and possibly other processes that are controlled by protein ubiquitination, play a role in many aspects of cellular function from the control of differentiation to intracellular trafficking [1,3,4]. Here we will review some of the results that implicate ubiquitin-dependent proteolysis in the control of cell growth and that indicate how perturbations of ubiquitin-dependent degradation of oncogene and tumor suppressor gene products may contribute to cell transformation and oncogenesis.
- Watkins JF, Sung P, Prakash L, Prakash S
- The Saccharomyces cerevisiae DNA repair gene RAD23 encodes a nuclear protein containing a ubiquitin-like domain required for biological function.
- Mol Cell Biol. 1993; 13: 7757-65
- Display abstract
In eukaryotes, the posttranslational conjugation of ubiquitin to various cellular proteins marks them for degradation. Interestingly, several proteins have been reported to contain ubiquitin-like (ub-like) domains that are in fact specified by the DNA coding sequences of the proteins. The biological role of the ub-like domain in these proteins is not known; however, it has been proposed that this domain functions as a degradation signal rendering the proteins unstable. Here, we report that the product of the Saccharomyces cerevisiae RAD23 gene, which is involved in excision repair of UV-damaged DNA, bears a ub-like domain at its amino terminus. This finding has presented an opportunity to define the functional significance of this domain. We show that deletion of the ub-like domain impairs the DNA repair function of RAD23 and that this domain can be functionally substituted by the authentic ubiquitin sequence. Surprisingly, RAD23 is highly stable, and the studies reported herein indicate that its ub-like domain does not mediate protein degradation. Thus, in RAD23 at least, the ub-like domain affects protein function in a nonproteolytic manner.
- Ozkaynak E, Finley D, Solomon MJ, Varshavsky A
- The yeast ubiquitin genes: a family of natural gene fusions.
- EMBO J. 1987; 6: 1429-39
- Display abstract
Ubiquitin is a 76-residue protein highly conserved among eukaryotes. Conjugation of ubiquitin to intracellular proteins mediates their selective degradation in vivo. We describe a family of four ubiquitin-coding loci in the yeast Saccharomyces cerevisiae. UB11, UB12 and UB13 encode hybrid proteins in which ubiquitin is fused to unrelated ('tail') amino acid sequences. The ubiquitin coding elements of UB11 and UB12 are interrupted at identical positions by non-homologous introns. UB11 and UB12 encode identical 52-residue tails, whereas UB13 encodes a different 76-residue tail. The tail amino acid sequences are highly conserved between yeast and mammals. Each tail contains a putative metal-binding, nucleic acid-binding domain of the form Cys-X2-4-Cys-X2-15-Cys-X2-4-Cys, suggesting that these proteins may function by binding to DNA. The fourth gene, UB14, encodes a polyubiquitin precursor protein containing five ubiquitin repeats in a head-to-tail, spacerless arrangement. All four ubiquitin genes are expressed in exponentially growing cells, while in stationary-phase cells the expression of UB11 and UB12 is repressed. The UB14 gene, which is strongly inducible by starvation, high temperatures and other stresses, contains in its upstream region strong homologies to the consensus 'heat shock box' nucleotide sequence. Elsewhere we show that the essential function of the UB14 gene is to provide ubiquitin to cells under stress.
- Metabolism (metabolic pathways involving proteins which contain this domain)
-
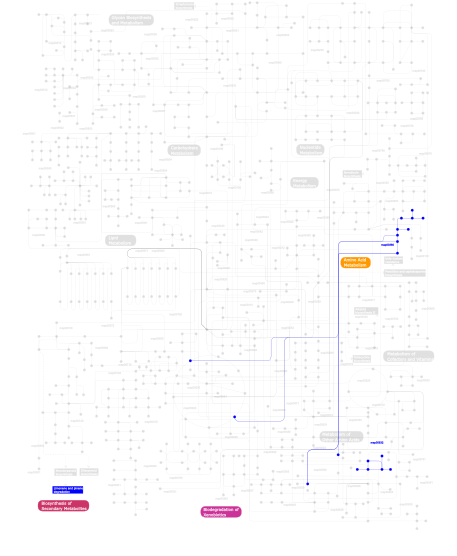
Click the image to view the interactive version of the map in iPath% proteins involved KEGG pathway ID Description 45.36 map03010 Ribosome 13.40 map05020 Parkinson's disease 13.40 map04120 Ubiquitin mediated proteolysis 11.34 map03320 PPAR signaling pathway 6.19 map05211 Renal cell carcinoma 5.15  map00380
map00380Tryptophan metabolism 2.06 map01040 Biosynthesis of unsaturated fatty acids 1.03 map03022 Basal transcription factors 1.03  map00632
map00632Benzoate degradation via CoA ligation 1.03 map00903 Limonene and pinene degradation This information is based on mapping of SMART genomic protein database to KEGG orthologous groups. Percentage points are related to the number of proteins with UBQ domain which could be assigned to a KEGG orthologous group, and not all proteins containing UBQ domain. Please note that proteins can be included in multiple pathways, ie. the numbers above will not always add up to 100%.
- Structure (3D structures containing this domain)
3D Structures of UBQ domains in PDB
PDB code Main view Title 1a5r 
STRUCTURE DETERMINATION OF THE SMALL UBIQUITIN-RELATED MODIFIER SUMO-1, NMR, 10 STRUCTURES 1aar 
STRUCTURE OF A DIUBIQUITIN CONJUGATE AND A MODEL FOR INTERACTION WITH UBIQUITIN CONJUGATING ENZYME (E2) 1bt0 
STRUCTURE OF UBIQUITIN-LIKE PROTEIN, RUB1 1c3t 
ROTAMER STRAIN AS A DETERMINANT OF PROTEIN STRUCTURAL SPECIFICITY 1cmx 
STRUCTURAL BASIS FOR THE SPECIFICITY OF UBIQUITIN C-TERMINAL HYDROLASES 1d3z 
UBIQUITIN NMR STRUCTURE 1euv 
X-RAY STRUCTURE OF THE C-TERMINAL ULP1 PROTEASE DOMAIN IN COMPLEX WITH SMT3, THE YEAST ORTHOLOG OF SUMO. 1f9j 
STRUCTURE OF A NEW CRYSTAL FORM OF TETRAUBIQUITIN 1fxt 
STRUCTURE OF A CONJUGATING ENZYME-UBIQUITIN THIOLESTER COMPLEX 1g6j 
STRUCTURE OF RECOMBINANT HUMAN UBIQUITIN IN AOT REVERSE MICELLES 1gjz 
Solution structure of a dimeric N-terminal fragment of human ubiquitin 1iyf 
Solution structure of ubiquitin-like domain of human parkin 1j8c 
Solution Structure of the Ubiquitin-like Domain of hPLIC-2 1l2n 
Smt3 Solution Structure 1lm8 
Structure of a HIF-1a-pVHL-ElonginB-ElonginC Complex 1lqb 
Crystal structure of a hydroxylated HIF-1 alpha peptide bound to the pVHL/elongin-C/elongin-B complex 1m94 
Solution Structure of the Yeast Ubiquitin-Like Modifier Protein Hub1 1mg8 
NMR structure of ubiquitin-like domain in murine Parkin 1nbf 
Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde 1ndd 
STRUCTURE OF NEDD8 1ogw 
Synthetic Ubiquitin with fluoro-Leu at 50 and 67 1oqy 
Structure of the DNA repair protein hHR23a 1otr 
Solution Structure of a CUE-Ubiquitin Complex 1p1a 
NMR structure of ubiquitin-like domain of hHR23B 1p3q 
Mechanism of Ubiquitin Recognition by the CUE Domain of VPS9 1p98 
High-resolution NMR structure of the Ubl-domain of HHR23A 1p9d 
High-resolution structure of the complex of HHR23A ubiquitin-like domain and the C-terminal ubiquitin-interacting motif of proteasome subunit S5a 1q0w 
Solution structure of Vps27 amino-terminal UIM-ubiquitin complex 1q5w 
Ubiquitin Recognition by Npl4 Zinc-Fingers 1qze 
HHR23a protein structure based on residual dipolar coupling data 1r4m 
APPBP1-UBA3-NEDD8, an E1-ubiquitin-like protein complex 1r4n 
APPBP1-UBA3-NEDD8, an E1-ubiquitin-like protein complex with ATP 1s1q 
TSG101(UEV) domain in complex with Ubiquitin 1sif 
Crystal structure of a multiple hydrophobic core mutant of ubiquitin 1tbe 
STRUCTURE OF TETRAUBIQUITIN SHOWS HOW MULTIUBIQUITIN CHAINS CAN BE FORMED 1tgz 
Structure of human Senp2 in complex with SUMO-1 1ttn 
Solution structure of the ubiquitin-like domain of human DC-UBP from dendritic cells 1u4a 
Solution structure of human SUMO-3 C47S 1ubi 
SYNTHETIC STRUCTURAL AND BIOLOGICAL STUDIES OF THE UBIQUITIN SYSTEM. PART 1 1ubq 
STRUCTURE OF UBIQUITIN REFINED AT 1.8 ANGSTROMS RESOLUTION 1ud7 
SOLUTION STRUCTURE OF THE DESIGNED HYDROPHOBIC CORE MUTANT OF UBIQUITIN, 1D7 1uel 
Solution structure of ubiquitin-like domain of hHR23B complexed with ubiquitin-interacting motif of proteasome subunit S5a 1uzx 
A complex of the Vps23 UEV with ubiquitin 1v5o 
Solution Structure of the Ubiquitin-like Domain from Mouse Hypothetical 1700011N24Rik Protein 1v5t 
Solution Structure of the Ubiquitin-like Domain from Mouse Hypothetical 8430435I17Rik Protein 1v80 
Solution structures of ubiquitin at 30 bar and 3 kbar 1v81 
Solution structures of ubiquitin at 30 bar and 3 kbar 1v86 
Solution structure of the ubiquitin domain from mouse D7Wsu128e protein 1vcb 
THE VHL-ELONGINC-ELONGINB STRUCTURE 1we6 
Solution structure of Ubiquitin-like domain in splicing factor AAL91182 1we7 
Solution structure of Ubiquitin-like domain in SF3a120 1wgd 
Solution structure of the Ubl-domain of Herp 1wgg 
Solution Structure of the N-terminal Ubiquitin-like Domain of Mouse Ubiquitin Specific Protease 14 (USP14) 1wh3 
Solution structure of C-terminal ubiquitin like domain of human 2'-5'-oligoadenylate synthetase-like protain (p59 OASL) 1wia 
Solution structure of mouse hypothetical ubiquitin-like protein BAB25500 1wm2 
Crystal structure of human SUMO-2 protein 1wm3 
Crystal structure of human SUMO-2 protein 1wr1 
The complex sturcture of Dsk2p UBA with ubiquitin 1wr6 
Crystal structure of GGA3 GAT domain in complex with ubiquitin 1wrd 
Crystal structure of Tom1 GAT domain in complex with ubiquitin 1wx7 
Solution Structure of the N-terminal Ubiquitin-like Domain in the Human Ubiquilin 3 (UBQLN3) 1wx8 
Solution Structure of the N-terminal Ubiquitin-like Domain in the 4931431F19Rik Protein 1wx9 
Solution Structure of the N-terminal Ubiquitin-like Domain in the Human BAT3 Protein 1wxv 
Solution structure of the ubiquitin domain of BCL-2 binding athanogene-1 1wy8 
Solution Structure of the N-terminal Ubiquitin-like Domain in Human Np95/ICBP90-like Ring Finger Protein (NIRF) 1wyw 
Crystal Structure of SUMO1-conjugated thymine DNA glycosylase 1wz0 
Solution Structure of Human SUMO-2 (SMT3B), a Ubiquitin-like Protein 1x1m 
Solution Structure of the N-terminal Ubiquitin-like Domain in Mouse Ubiquitin-like Protein SB132 1xd3 
Crystal structure of UCHL3-UbVME complex 1xqq 
Simultaneous determination of protein structure and dynamics 1xt9 
Crystal Structure of Den1 in complex with Nedd8 1y8r 
SUMO E1 ACTIVATING ENZYME SAE1-SAE2-SUMO1-MG-ATP COMPLEX 1yd8 
COMPLEX OF HUMAN GGA3 GAT DOMAIN AND UBIQUITIN 1yiw 
X-ray Crystal Structure of a Chemically Synthesized Ubiquitin 1yj1 
X-ray Crystal Structure of a Chemically Synthesized [D-Gln35]Ubiquitin 1yqb 
Human Ubiquilin 3 1yx5 
Solution Structure of S5a UIM-1/Ubiquitin Complex 1yx6 
Solution Structure of S5a UIM-2/Ubiquitin Complex 1z2m 
Crystal Structure of ISG15, the Interferon-Induced Ubiquitin Cross Reactive Protein 1z5s 
Crystal structure of a complex between UBC9, SUMO-1, RANGAP1 and NUP358/RANBP2 1zgu 
Solution structure of the human Mms2-Ubiquitin complex 1zkh 
Solution structure of a human ubiquitin-like domain in SF3A1 1zw7 
Elimination of the C-cap in Ubiquitin Structure, Dynamics and Thermodynamic Consequences 2asq 
Solution Structure of SUMO-1 in Complex with a SUMO-binding Motif (SBM) 2awt 
Solution Structure of Human Small Ubiquitin-Like Modifier Protein Isoform 2 (SUMO-2) 2ayo 
Structure of USP14 bound to ubquitin aldehyde 2bf8 
Crystal structure of SUMO modified ubiquitin conjugating enzyme E2- 25K 2bgf 
NMR structure of Lys48-linked di-ubiquitin using chemical shift perturbation data together with RDCs and 15N-relaxation data 2bkr 
NEDD8 NEDP1 complex 2bwe 
The crystal structure of the complex between the UBA and UBL domains of Dsk2 2bwf 
Crystal sturcture of the UBL domain of Dsk2 from S. cerevisiae 2c7m 
human Rabex-5 residues 1-74 in complex with Ubiquitin 2c7n 
Human Rabex-5 residues 1-74 in complex with Ubiquitin 2c9w 
CRYSTAL STRUCTURE OF SOCS-2 IN COMPLEX WITH ELONGIN-B AND ELONGIN-C AT 1.9A RESOLUTION 2ckh 
SENP1-SUMO2 complex 2d07 
Crystal Structure of SUMO-3-modified Thymine-DNA Glycosylase 2d3g 
Double sided ubiquitin binding of Hrs-UIM 2den 
Solution Structure of the Ubiquitin-Associated Domain of Human BMSC-UbP and its Complex with Ubiquitin 2dx5 
The complex structure between the mouse EAP45-GLUE domain and ubiquitin 2dzi 
2DZI/Solution Structure of the N-terminal Ubiquitin-like Domain in Human Ubiquitin-like Protein 4A (GDX) 2eke 
Structure of a SUMO-binding-motif mimic bound to Smt3p-Ubc9p: conservation of a noncovalent Ubiquitin-like protein-E2 complex as a platform for selective interactions within a SUMO pathway 2faz 
Ubiquitin-Like Domain of Human Nuclear Zinc Finger Protein NP95 2fcm 
X-ray Crystal Structure of a Chemically Synthesized [D-Gln35]Ubiquitin with a Cubic Space Group 2fcn 
X-ray Crystal Structure of a Chemically Synthesized [D-Val35]Ubiquitin with a Cubic Space Group 2fcq 
X-ray Crystal Structure of a Chemically Synthesized Ubiquitin with a Cubic Space Group 2fcs 
X-ray Crystal Structure of a Chemically Synthesized [L-Gln35]Ubiquitin with a Cubic Space Group 2fid 
Crystal Structure of a Bovine Rabex-5 fragment complexed with ubiquitin 2fif 
Crystal Structure of a Bovine Rabex-5 fragment complexed with ubiquitin 2fnj 
Crystal structure of a B30.2/SPRY domain-containing protein GUSTAVUS in complex with Elongin B and Elongin C 2fuh 
Solution Structure of the UbcH5c/Ub Non-covalent Complex 2g3q 
Solution Structure of Ede1 UBA-ubiquitin complex 2g45 
Co-crystal structure of znf ubp domain from the deubiquitinating enzyme isopeptidase T (isot) in complex with ubiquitin 2g4d 
Crystal structure of human SENP1 mutant (C603S) in complex with SUMO-1 2gbj 
Crystal Structure of the 9-10 8 Glycine Insertion Mutant of Ubiquitin. 2gbk 
Crystal Structure of the 9-10 MoaD Insertion Mutant of Ubiquitin 2gbm 
Crystal Structure of the 35-36 8 Glycine Insertion Mutant of Ubiquitin 2gbn 
Crystal Structure of the 35-36 8 Glycine Insertion Mutant of Ubiquitin 2gbr 
Crystal Structure of the 35-36 MoaD Insertion Mutant of Ubiquitin 2gmi 
Mms2/Ubc13~Ubiquitin 2hd5 
USP2 in complex with ubiquitin 2hj8 
Solution NMR structure of the C-terminal domain of the interferon alpha-inducible ISG15 protein from Homo sapiens. Northeast Structural Genomics target HR2873B 2hth 
Structural basis for ubiquitin recognition by the human EAP45/ESCRT-II GLUE domain 2ibi 
Covalent Ubiquitin-USP2 Complex 2io0 
Crystal structure of human Senp2 in complex with preSUMO-2 2io1 
Crystal structure of human Senp2 in complex with preSUMO-3 2io2 
Crystal structure of human Senp2 in complex with RanGAP1-SUMO-1 2io3 
Crystal structure of human Senp2 in complex with RanGAP1-SUMO-2 2iy0 
SENP1 (mutant) SUMO1 RanGAP 2iy1 
SENP1 (mutant) full length SUMO1 2iyd 
SENP1 covalent complex with SUMO-2 2izv 
CRYSTAL STRUCTURE OF SOCS-4 IN COMPLEX WITH ELONGIN-B AND ELONGIN-C AT 2.55A RESOLUTION 2j7q 
Crystal structure of the ubiquitin-specific protease encoded by murine cytomegalovirus tegument protein M48 in complex with a ubquitin-based suicide substrate 2jf5 
crystal structure of Lys63-linked di-ubiquitin 2jri 
Solution structure of the Josephin domain of Ataxin-3 in complex with ubiquitin molecule. 2jt4 
Solution Structure of the Sla1 SH3-3-Ubiquitin Complex 2jvc 
NMR solution structure of ubiquitin like protein 2jwz 
Mutations in the hydrophobic core of ubiquitin differentially affect its recognition by receptor proteins 2jy6 
Solution structure of the complex of ubiquitin and ubiquilin 1 UBA domain 2jz3 
SOCS box elonginBC ternary complex 2jzz 
Solid-State NMR Structure of Microcrystalline Ubiquitin 2k1f 
SUMO-3 from Drosophila melanogaster (dsmt3) 2k25 
Automated NMR Structure of the UBB by FAPSY 2k39 
Recognition dynamics up to microseconds revealed from RDC derived ubiquitin ensemble in solution 2k6d 
CIN85 Sh3-C domain in complex with ubiquitin 2k8b 
Solution structure of PLAA family ubiquitin binding domain (PFUC) cis isomer in complex with ubiquitin 2k8c 
Solution structure of PLAA family ubiquitin binding domain (PFUC) trans isomer in complex with ubiquitin 2k8h 
Solution structure of SUMO from Trypanosoma brucei 2kan 
Solution NMR structure of ubiquitin-like domain of Arabidopsis thaliana protein At2g32350. Northeast Structural Genomics Consortium target AR3433A 2kd0 
NMR solution structure of O64736 protein from Arabidopsis thaliana. Northeast Structural Genomics Consortium MEGA Target AR3445A 2kde 
NMR structure of major S5a (196-306):K48 linked diubiquitin species 2kdf 
NMR structure of minor S5a (196-306):K48 linked diubiquitin species 2kdi 
Solution structure of a Ubiquitin/UIM fusion protein 2khw 
Solution Structure of the human Polymerase iota UBM2-Ubiquitin Complex 2kjh 
NMR based structural model of the UBCH8-UBIQUITIN complex 2kk8 
NMR Solution Structure of a Putative Uncharacterized Protein obtained from Arabidopsis thaliana: Northeast Structural Genomics Consortium target AR3449A 2klc 
NMR solution structure of human ubiquitin-like domain of ubiquilin 1, Northeast Structural Genomics Consortium (NESG) target HT5A 2klg 
PERE NMR structure of ubiquitin 2kn5 
A Correspondence Between Solution-State Dynamics of an Individual Protein and the Sequence and Conformational Diversity of its Family 2knb 
Solution NMR structure of the parkin Ubl domain in complex with the endophilin-A1 SH3 domain 2ko3 
Nedd8 solution structure 2kox 
NMR residual dipolar couplings identify long range correlated motions in the backbone of the protein ubiquitin 2kqs 
Phosphorylation of SUMO-interacting motif by CK2 enhances Daxx SUMO binding activity 2ktf 
Solution NMR structure of human polymerase iota UBM2 in complex with ubiquitin 2kwu 
Solution Structure of UBM2 of murine Polymerase iota in Complex with Ubiquitin 2kwv 
Solution Structure of UBM1 of murine Polymerase iota in Complex with Ubiquitin 2kx0 
the solution structure of UBB+1, frameshift mutant of ubiquitin B 2kx3 
The solution structure of the mutant of UBL domain of UBLCP1, I5M 2l00 
Solution structure of the non-covalent complex of the ZNF216 A20 domain with ubiquitin 2l0f 
Solution NMR structure of human polymerase iota UBM2 (P692A mutant) in complex with ubiquitin 2l0t 
Solution structure of the complex of ubiquitin and the VHS domain of Stam2 2l3z 
Proton-Detected 4D DREAM Solid-State NMR Structure of Ubiquitin 2l76 
Solution NMR structure of human NFATC2IP ubiquitin-like domain, NFATC2IP_244_338, NESG target HT65A/OCSP target hs00387_244_338/SGC-toronto 2l7r 
Solution NMR structure of N-terminal Ubiquitin-like domain of FUBI, a ribosomal protein S30 precursor from Homo sapiens. NorthEast Structural Genomics consortium (NESG) target HR6166 2las 
Molecular Determinants of Paralogue-Specific SUMO-SIM Recognition 2ld9 
Backbone Structure of Ubiquitin determined using Backbone amide NOEs and Backbone N-H and N-C RDCs 2lgd 
The high resolution structure of ubiquitin like domain of UBLCP1 2lj5 
Description of the Structural fluctuations of proteins from structure-based calculations of Residual dipolar couplings 2lrw 
Solution structure of a ubiquitin-like protein from Trypanosoma brucei 2lvo 
Structure of the gp78CUE domain bound to monubiquitin 2lvp 
gp78CUE domain bound to the distal ubiquitin of K48-linked diubiquitin 2lvq 
gp78CUE domain bound to the proximal ubiquitin of K48-linked diubiquitin 2lwp 
The NMR solution structure of the the ubiquitin homology domain of mouse BAG-1 2lxa 
Solution structure of the Get5 ubiquitin-like domain 2lxc 
Solution structure of the complex between the Sgt2 homodimerization domain and the Get5 UBL domain 2lz6 
Distinct ubiquitin binding modes exhibited by sh3 domains: molecular determinants and functional implications 2m0x 
Solution structure of U14Ub1, an engineered ubiquitin variant with increased affinity for USP14 2m17 
ubiquitin-like domain-containing C-terminal domain phosphatase (UBLCP1) 2m8s 
NMR Structure of the Cytoplasmic Tail of the Membrane Form of Heparin-binding EGF-like Growth Factor (proHB-EGF-CT) Complexed with the Ubiquitin Homology Domain of Bcl-2-associated Athanogene 1 from Mus musculus (mBAG-1-UBH) 2ma9 
HIV-1 Vif SOCS-box and Elongin BC solution structure 2mbb 
2MBB 2mbe 
2MBE 2mbh 
NMR structure of EKLF(22-40)/Ubiquitin Complex 2mbo 
K11-linked Diubiquitin average solution structure at pH 6.8, 0 mM NaCl 2mbq 
K11-linked Diubiquitin average solution structure at pH 6.8, 150 mM NaCl 2mcn 
Distinct ubiquitin binding modes exhibited by SH3 domains: molecular determinants and functional implications 2mi8 
Solution structure of lysine-free (K0) ubiquitin 2mj5 
Structure of the UBA Domain of Human NBR1 in Complex with Ubiquitin 2mjb 
Solution nmr structure of ubiquitin refined against dipolar couplings in 4 media 2mlb 
2MLB 2mor 
2MOR 2mp2 
2MP2 2mqj 
2MQJ 2mre 
2MRE 2mro 
2MRO 2msg 
2MSG 2mur 
2MUR 2mw5 
2MW5 2mws 
2MWS 2n13 
2N13 2n1a 
2N1A 2n1v 
2N1V 2n1w 
2N1W 2n2k 
2N2K 2n3u 
2N3U 2n3v 
2N3V 2n3w 
2N3W 2n4f 
2N4F 2n7k 
2N7K 2n9e 
2N9E 2n9p 
2N9P 2nbd 
2NBD 2nbe 
2NBE 2nbu 
2NBU 2nbv 
2NBV 2nbw 
2NBW 2nr2 
The MUMO (minimal under-restraining minimal over-restraining) method for the determination of native states ensembles of proteins 2nvu 
Structure of APPBP1-UBA3~NEDD8-NEDD8-MgATP-Ubc12(C111A), a trapped ubiquitin-like protein activation complex 2o6v 
Crystal structure and solution NMR studies of Lys48-linked tetraubiquitin at neutral pH 2ojr 
Structure of ubiquitin solved by SAD using the Lanthanide-Binding Tag 2oob 
crystal structure of the UBA domain from Cbl-b ubiquitin ligase in complex with ubiquitin 2pe6 
Non-covalent complex between human SUMO-1 and human Ubc9 2pe9 
NMR Based Structure of the Open Conformation of LYS48-Linked Di-UBiquitin Using Experimental Global Rotational Diffusion Tensor from NMR Relaxation Measurements 2pea 
NMR Based Structure of the Closed Conformation of LYS48-Linked Di-Ubiquitin Using Experimental Global Rotational Diffusion Tensor from NMR Relaxation Measurements 2qho 
Crystal structure of the UBA domain from EDD ubiquitin ligase in complex with ubiquitin 2rpq 
Solution Structure of a SUMO-interacting motif of MBD1-containing chromatin-associated factor 1 bound to SUMO-3 2rr9 
The solution structure of the K63-Ub2:tUIMs complex 2rsu 
Alternative structure of Ubiquitin 2ru6 
The pure alternative state of ubiquitin 2uyz 
Non-covalent complex between Ubc9 and SUMO1 2vrr 
Structure of SUMO modified Ubc9 2w9n 
crystal structure of linear di-ubiquitin 2wdt 
Crystal structure of Plasmodium falciparum UCHL3 in complex with the suicide inhibitor UbVME 2wwz 
TAB2 NZF DOMAIN IN COMPLEX WITH Lys63-linked di-ubiquitin, P212121 2wx0 
TAB2 NZF DOMAIN IN COMPLEX WITH Lys63-linked di-ubiquitin, P21 2wx1 
TAB2 NZF DOMAIN IN COMPLEX WITH Lys63-linked tri-ubiquitin, P212121 2wyq 
THE CRYSTAL STRUCTURE OF THE UBIQUITIN-LIKE (UBL) DOMAIN OF HHR23A ( HUMAN HOMOLOGUE A OF RAD23) 2xbb 
Nedd4 HECT:Ub complex 2xew 
Crystal structure of K11-linked diubiquitin 2xk5 
Crystal structure of K6-linked diubiquitin 2y5b 
Structure of USP21 in complex with linear diubiquitin-aldehyde 2z59 
Complex Structures of Mouse Rpn13 (22-130aa) and ubiquitin 2zcb 
Crystal Structure of ubiquitin P37A/P38A 2zcc 
Ubiquitin crystallized under high pressure 2zeq 
Crystal structure of ubiquitin-like domain of murine Parkin 2znv 
Crystal structure of human AMSH-LP DUB domain in complex with Lys63-linked ubiquitin dimer 2zvn 
NEMO CoZi domain incomplex with diubiquitin in P212121 space group 2zvo 
NEMO CoZi domain in complex with diubiquitin in C2 space group 3a1q 
Crystal structure of the mouse RAP80 UIMs in complex with Lys63-linked di-ubiquitin 3a33 
UbcH5b~Ubiquitin Conjugate 3a9j 
Crystal structure of the mouse TAB2-NZF in complex with Lys63-linked di-ubiquitin 3a9k 
Crystal structure of the mouse TAB3-NZF in complex with Lys63-linked di-ubiquitin 3ai5 
Crystal structure of yeast enhanced green fluorescent protein-ubiquitin fusion protein 3alb 
Cyclic Lys48-linked tetraubiquitin 3aul 
Crystal structure of wild-type Lys48-linked diubiquitin in an open conformation 3axc 
Crystal structure of linear diubiquitin 3b08 
Crystal structure of the mouse HOIL1-L-NZF in complex with linear di-ubiquitin 3b0a 
Crystal structure of the mouse HOIL1-L-NZF in complex with linear di-ubiquitin 3b1l 
Crystal Structure of parkin ubiquitin-like domain R33Q mutant 3by4 
Structure of Ovarian Tumor (OTU) domain in complex with Ubiquitin 3c0r 
Structure of Ovarian Tumor (OTU) domain in complex with Ubiquitin 3cmm 
Crystal Structure of the Uba1-Ubiquitin Complex 3dbh 
Structural Dissection of a Gating Mechanism Preventing Misactivation of Ubiquitin by NEDD8's E1 (APPBP1-UBA3Arg190Ala-NEDD8Ala72Arg) 3dbl 
Structural Dissection of a Gating Mechanism Preventing Misactivation of Ubiquitin by NEDD8's E1 (APPBP1-UBA3Arg190wt-NEDD8Ala72Gln) 3dbr 
Structural Dissection of a Gating Mechanism Preventing Misactivation of Ubiquitin by NEDD8's E1 (APPBP1-UBA3Arg190Gln-NEDD8Ala72Arg) 3dcg 
Crystal Structure of the HIV Vif BC-box in Complex with Human ElonginB and ElonginC 3dqv 
Structural Insights into NEDD8 Activation of Cullin-RING Ligases: Conformational Control of Conjugation 3dvg 
Crystal structure of K63-specific fab Apu.3A8 bound to K63-linked di-ubiquitin 3dvn 
Crystal structure of K63-specific fab Apu2.16 bound to K63-linked di-ubiquitin 3eec 
X-ray structure of human ubiquitin Cd(II) adduct 3efu 
X-ray structure of human ubiquitin-Hg(II) adduct 3ehv 
X-ray structure of human ubiquitin Zn(II) adduct 3gzn 
Structure of NEDD8-activating enzyme in complex with NEDD8 and MLN4924 3h1u 
Structure of ubiquitin in complex with Cd ions 3h7p 
Crystal structure of K63-linked di-ubiquitin 3h7s 
Crystal structures of K63-linked di- and tri-ubiquitin reveal a highly extended chain architecture 3hm3 
The Structure and conformation of Lys-63 linked tetra-ubiquitin 3i3t 
Crystal structure of covalent ubiquitin-USP21 complex 3ifw 
Crystal structure of the S18Y variant of ubiquitin carboxy terminal hydrolase L1 bound to ubiquitin vinylmethylester. 3ihp 
Covalent Ubiquitin-Usp5 Complex 3ix6 
Crystal structure of Thymidylate synthase thyA from Brucella melitensis 3j6x 
3J6X 3j6y 
3J6Y 3j77 
3J77 3j78 
3J78 3j79 
3J79 3j7o 
3J7O 3j7p 
3J7P 3j7q 
3J7Q 3j7r 
3J7R 3j80 
3J80 3j81 
3J81 3j92 
3J92 3jam 
3JAM 3jap 
3JAP 3jaq 
3JAQ 3jsv 
Crystal structure of mouse NEMO CoZi in complex with Lys63-linked di-ubiquitin 3jvz 
E2~Ubiquitin-HECT 3jw0 
E2~Ubiquitin-HECT 3k9o 
The crystal structure of E2-25K and UBB+1 complex 3k9p 
The crystal structure of E2-25K and ubiquitin complex 3kvf 
Crystal structure of the I93M mutant of ubiquitin carboxy terminal hydrolase L1 bound to ubiquitin vinylmethylester 3kw5 
Crystal structure of ubiquitin carboxy terminal hydrolase L1 bound to ubiquitin vinylmethylester 3kyc 
Human SUMO E1 complex with a SUMO1-AMP mimic 3kyd 
Human SUMO E1~SUMO1-AMP tetrahedral intermediate mimic 3l0w 
Structure of split monoubiquitinated PCNA with ubiquitin in position two 3l10 
Structure of split monoubiquitinated PCNA with ubiquitin in position one 3ldz 
Crystal structure of human STAM1 VHS domain in complex with ubiquitin 3m3j 
A new crystal form of Lys48-linked diubiquitin 3m62 
Crystal structure of Ufd2 in complex with the ubiquitin-like (UBL) domain of Rad23 3m63 
Crystal structure of Ufd2 in complex with the ubiquitin-like (UBL) domain of Dsk2 3mhs 
Structure of the SAGA Ubp8/Sgf11/Sus1/Sgf73 DUB module bound to ubiquitin aldehyde 3mtn 
Usp21 in complex with a ubiquitin-based, USP21-specific inhibitor 3n30 
Crystal Structure of cubic Zn3-hUb (human ubiquitin) adduct 3n32 
The crystal structure of human Ubiquitin adduct with Zeise's salt 3n3k 
The catalytic domain of USP8 in complex with a USP8 specific inhibitor 3nhe 
High Resolution Structure (1.26A) of USP2a in Complex with Ubiquitin 3nob 
Structure of K11-linked di-ubiquitin 3ns8 
Crystal structure of an open conformation of Lys48-linked diubiquitin at pH 7.5 3o65 
Crystal structure of a Josephin-ubiquitin complex: Evolutionary restraints on ataxin-3 deubiquitinating activity 3ofi 
Crystal structure of human insulin-degrading enzyme in complex with ubiquitin 3oj3 
Crystal structure of the A20 ZnF4 and ubiquitin complex 3oj4 
Crystal structure of the A20 ZnF4, ubiquitin and UbcH5A complex 3olm 
Structure and Function of a Ubiquitin Binding Site within the Catalytic Domain of a HECT Ubiquitin Ligase 3ons 
Crystal structure of Human Ubiquitin in a new crystal form 3pge 
Structure of sumoylated PCNA 3phd 
Crystal structure of human HDAC6 in complex with ubiquitin 3phw 
OTU Domain of Crimean Congo Hemorrhagic Fever Virus in complex with Ubiquitin 3phx 
OTU Domain of Crimean Congo Hemorrhagic Fever Virus in complex with ISG15 3plu 
Structure of Hub-1 protein in complex with Snu66 peptide (HINDI) 3plv 
Structure of Hub-1 protein in complex with Snu66 peptide (HINDII) 3prm 
Structural analysis of a viral OTU domain protease from the Crimean-Congo Hemorrhagic Fever virus in complex with human ubiquitin 3prp 
Structural analysis of a viral OTU domain protease from the Crimean-Congo Hemorrhagic Fever virus in complex with human ubiquitin 3pse 
Structure of a viral OTU domain protease bound to interferon-stimulated gene 15 (ISG15) 3pt2 
Structure of a viral OTU domain protease bound to Ubiquitin 3ptf 
X-ray structure of the non-covalent complex between UbcH5A and Ubiquitin 3q3f 
Engineering Domain-Swapped Binding Interfaces by Mutually Exclusive Folding: Insertion of Ubiquitin into position 103 of Barnase 3qht 
Crystal Structure of the Monobody ySMB-1 bound to yeast SUMO 3r66 
Crystal structure of human ISG15 in complex with NS1 N-terminal region from influenza virus B, Northeast Structural Genomics Consortium Target IDs HX6481, HR2873, and OR2 3rt3 
Complex of influenza virus protein with host anti-viral factor 3rul 
New strategy to analyze structures of glycopeptide-target complexes 3rzw 
Crystal Structure of the Monobody ySMB-9 bound to human SUMO1 3sdl 
Crystal structure of human ISG15 in complex with NS1 N-terminal region from influenza B virus, Northeast Structural Genomics Consortium Target IDs HX6481, HR2873, and OR2 3shq 
Crystal Structure of UBLCP1 3tbl 
Structure of Mono-ubiquitinated PCNA: Implications for DNA Polymerase Switching and Okazaki Fragment Maturation 3tix 
Crystal structure of the Chp1-Tas3 complex core 3tmp 
The catalytic domain of human deubiquitinase DUBA in complex with ubiquitin aldehyde 3u30 
Crystal structure of a linear-specific Ubiquitin fab bound to linear ubiquitin 3uf8 
Crystal structure of a SMT fusion Peptidyl-prolyl cis-trans isomerase with a G95A surface mutation from Burkholderia pseudomallei complexed with FK506 3ugb 
UbcH5c~Ubiquitin Conjugate 3uin 
Complex between human RanGAP1-SUMO2, UBC9 and the IR1 domain from RanBP2 3uio 
Complex between human RanGAP1-SUMO2, UBC9 and the IR1 domain from RanBP2 containing IR2 Motif II 3uip 
Complex between human RanGAP1-SUMO1, UBC9 and the IR1 domain from RanBP2 containing IR2 Motif II 3uqa 
Crystal structure of a SMT fusion Peptidyl-prolyl cis-trans isomerase with surface mutation A54E from Burkholderia pseudomallei complexed with FK506 3uqb 
Crystal structure of a SMT Fusion PEPTIDYL-PROLYL CIS-TRANS ISOMERASE with surface mutation D44G from Burkholderia pseudomallei complexed with FK506 3v60 
Structure of S. cerevisiae PCNA conjugated to SUMO on lysine 164 3v61 
Structure of S. cerevisiae PCNA conjugated to SUMO on lysine 164 3v62 
Structure of the S. cerevisiae Srs2 C-terminal domain in complex with PCNA conjugated to SUMO on lysine 164 3v6c 
Crystal Structure of USP2 in complex with mutated ubiquitin 3v6e 
Crystal Structure of USP2 and a mutant form of Ubiquitin 3v7o 
Crystal structure of the C-terminal domain of Ebola virus VP30 (strain Reston-89) 3vaw 
Crystal structure of a smt fusion peptidyl-prolyl cis-trans isomerase with surface mutation v3i from burkholderia pseudomallei complexed with fk506 3vdz 
Tailoring Encodable Lanthanide-Binding Tags as MRI Contrast Agents: xq-dSE3-Ubiquitin at 2.4 Angstroms 3vfk 
The structure of monodechloro-teicoplanin in complex with its ligand, using ubiquitin as a ligand carrier 3vht 
Crystal structure of GFP-Wrnip1 UBZ domain fusion protein in complex with ubiquitin 3vuw 
Crystal structure of A20 ZF7 in complex with linear ubiquitin, form I 3vux 
Crystal structure of A20 ZF7 in complex with linear ubiquitin, form II 3vuy 
Crystal structure of A20 ZF7 in complex with linear tetraubiquitin 3wwq 
3WWQ 3wxe 
3WXE 3wxf 
3WXF 3wxg 
3WXG 3zdm 
Crystal structure of the Sgt2 N domain and the Get5 UBL domain complex 3zkj 
Crystal Structure of Ankyrin Repeat and Socs Box-Containing Protein 9 (Asb9) in Complex with Elonginb and Elonginc 3zlz 
Lys6-linked tri-ubiquitin 3zng 
Ankyrin repeat and SOCS-box protein 9 (ASB9) in complex with ElonginB and ElonginC 3znh 
Crimean Congo Hemorrhagic Fever Virus OTU domain in complex with ubiquitin-propargyl. 3zni 
Structure of phosphoTyr363-Cbl-b - UbcH5B-Ub - ZAP-70 peptide complex 3znz 
Crystal structure of OTULIN OTU domain (C129A) in complex with Met1- di ubiquitin 3zo5 
Structure of SENP2-Loop1 in complex with preSUMO-2 3zrc 
pVHL54-213-EloB-EloC complex (4R)-4-HYDROXY-1-[(3-METHYLISOXAZOL-5-YL)ACETYL]-N-[4-(1,3-OXAZOL-5-YL)BENZYL]-L-PROLINAMIDE bound 3zrf 
pVHL54-213-EloB-EloC complex_apo 3ztc 
pVHL54-213-EloB-EloC complex _ (2S,4R)-N-((1,1'-biphenyl)-4-ylmethyl)- 4-hydroxy-1-(2-(3-methylisoxazol-5-yl)acetyl)pyrrolidine-2- carboxamide 3ztd 
pVHL54-213-EloB-EloC complex _ methyl 4-(((2S,4R)-4-hydroxy-1-(2-(3- methylisoxazol-5-yl)acetyl)pyrrolidine-2-carboxamido)methyl)benzoate 3zun 
pVHL54-213-EloB-EloC complex_(2S,4R)-4-hydroxy-1-(2-(3-methylisoxazol- 5-yl)acetyl)-N-(4-nitrobenzyl)pyrrolidine-2-carboxamide bound 4a20 
Crystal structure of the Ubl domain of Mdy2 (Get5) at 1.78A 4adx 
The Cryo-EM Structure of the Archaeal 50S Ribosomal Subunit in Complex with Initiation Factor 6 4ajy 
von Hippel-Lindau protein-ElonginB-ElonginC complex, bound to Hif1- alpha peptide 4ap4 
Rnf4 - ubch5a - ubiquitin heterotrimeric complex 4asw 
Structure of the complex between the N-terminal dimerisation domain of Sgt2 and the UBL domain of Get5 4auq 
Structure of BIRC7-UbcH5b-Ub complex. 4awj 
pVHL:EloB:EloC complex, in complex with capped Hydroxyproline 4b95 
pVHL-EloB-EloC complex_(2S,4R)-1-(2-chlorophenyl)carbonyl-N-[(4-chlorophenyl)methyl]-4-oxidanyl-pyrrolidine-2-carboxamide bound 4b9k 
pVHL-ELOB-ELOC complex_(2S,4R)-1-(3-amino-2-methylbenzoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide bound 4bbn 
NEDD4 HECT-Ub:Ub complex 4bkg 
crystal structure of human diSUMO-2 4bks 
von Hippel Lindau protein:ElonginB:ElonginC complex, in complex with (2S,4R)-1-ethanoyl-N-[[4-(1,3-oxazol-5-yl)phenyl]methyl]-4-oxidanyl-pyrrolidine-2-carboxamide 4bkt 
von Hippel Lindau protein:ElonginB:ElonginC complex, in complex with (2S,4R)-N-methyl-1-[2-(3-methyl-1,2-oxazol-5-yl)ethanoyl]-4-oxidanyl-pyrrolidine-2-carboxamide 4bos 
Structure of OTUD2 OTU domain in complex with Ubiquitin K11-linked peptide 4boz 
Structure of OTUD2 OTU domain in complex with K11-linked di ubiquitin 4bvu 
Structure of Shigella effector OspG in complex with host UbcH5c- Ubiquitin conjugate 4d5l 
4D5L 4d5y 
4D5Y 4d61 
4D61 4d67 
4D67 4da1 
Crystal structure of branched-chain alpha-ketoacid dehydrogenase phosphatase with Mg (II) ions at the active site 4ddg 
Crystal structure of human OTUB1/UbcH5b~Ub/Ub 4ddi 
Crystal structure of human OTUB1/UbcH5b~Ub/Ub 4dhj 
The structure of a ceOTUB1 ubiquitin aldehyde UBC13~Ub complex 4dhz 
The structure of h/ceOTUB1-ubiquitin aldehyde-UBC13~Ub 4dwf 
Crystal structure of a HLA-B associated transcript 3 (BAT3) from Homo sapiens at 1.80 A resolution 4eew 
Crystal structure of the Ubl domain of BAG6 4f8c 
Structure of the Cif:Nedd8 complex - Yersinia pseudotuberculosis Cycle Inhibiting Factor in complex with human Nedd8 4fbj 
Structure of the Cif:Nedd8 complex - Photorhabdus luminescens Cycle Inhibiting Factor in complex with human Nedd8 4fjv 
Crystal Structure of Human Otubain2 and Ubiquitin Complex 4fn2 
Crystal structure of a SMT fusion Peptidyl-prolyl cis-trans isomerase with surface mutation D44G from Burkholderia pseudomallei complexed with CJ37 4g50 
Crystal structure of a SMT fusion Peptidyl-prolyl cis-trans isomerase with surface mutation D44G from Burkholderia pseudomallei complexed with CJ168 4ggq 
Crystal structure of a SMT fusion Peptidyl-prolyl cis-trans isomerase from Burkholderia pseudomallei complexed with CJ40 4giv 
Crystal structure of a SMT fusion Peptidyl-prolyl cis-trans isomerase with surface mutation D44G from Burkholderia pseudomallei complexed with CJ183 4goc 
Crystal structure of the Get5 ubiquitin-like domain 4gsw 
Crystal structure of ubiquitin from Entamoeba histolytica to 2.15 Angstrom 4gu2 
Crystal structure of ubiquitin from Entamoeba histolytica to 1.35 Angstrom 4hcn 
Crystal structure of Burkholderia pseudomallei effector protein CHBP in complex with ubiquitin 4hcp 
crystal structure of Burkholderia pseudomallei effector protein chbp in complex with nedd8 4hjk 
U7Ub7 Disulfide variant 4hk2 
U7Ub25.2540 4hxd 
Diversity of ubiquitin and ISG15 specificity amongst nairoviruses viral ovarian tumor domain proteases 4i6l 
Crystal structure of OTUB1 in complex with ubiquitin variant 4i6n 
Crystal structure of Trichinella spiralis UCH37 catalytic domain bound to Ubiquitin vinyl methyl ester 4ig7 
Crystal structure of Trichinella spiralis UCH37 bound to Ubiquitin vinyl methyl ester 4ii2 
Crystal structure of Ubiquitin activating enzyme 1 (Uba1) in complex with the Ub E2 Ubc4, ubiquitin, and ATP/Mg 4ii3 
Crystal structure of S. pombe Ubiquitin activating enzyme 1 (Uba1) in complex with ubiquitin and ATP/Mg 4ium 
Equine arteritis virus papain-like protease 2 (PLP2) covalently bound to ubiquitin 4jgh 
Structure of the SOCS2-Elongin BC complex bound to an N-terminal fragment of Cullin5 4jio 
Bro1 V domain and ubiquitin 4jqw 
Crystal Structure of a Complex of NOD1 CARD and Ubiquitin 4k1r 
Crystal structure of Schizosaccharomyces pombe sst2 catalytic domain and Ubiquitin 4k7s 
Crystal structure of Zn2-hUb (human ubiquitin) adduct from a solution 35 mM zinc acetate/1.3 mM hUb 4k7u 
Crystal structure of Zn2.3-hUb (human ubiquitin) adduct from a solution 70 mM zinc acetate/1.3 mM hUb 4k7w 
Crystal structure of Zn3-hUb(human ubiquitin) adduct from a solution 100 mM zinc acetate/1.3 mM hUb 4k95 
Crystal Structure of Parkin 4ksk 
Gumby/Fam105B in complex with ubiquitin 4ksl 
Gumby/Fam105B in complex with linear di-ubiquitin 4kzx 
Rabbit 40S ribosomal subunit in complex with eIF1. 4kzy 
Rabbit 40S ribosomal subunit in complex with eIF1 and eIF1A. 4kzz 
Rabbit 40S ribosomal subunit in complex with mRNA, initiator tRNA and eIF1A 4lcd 
Structure of an Rsp5xUbxSna3 complex: Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3 4ldt 
The structure of h/ceOTUB1-ubiquitin aldehyde-UBCH5B~Ub 4ljo 
Structure of an active ligase (HOIP)/ubiquitin transfer complex 4ljp 
Structure of an active ligase (HOIP-H889A)/ubiquitin transfer complex 4m0w 
Crystal Structure of SARS-CoV papain-like protease C112S mutant in complex with ubiquitin 4mdk 
Cdc34-ubiquitin-CC0651 complex 4mm3 
4MM3 4msm 
4MSM 4msq 
4MSQ 4n9f 
Crystal structure of the Vif-CBFbeta-CUL5-ElOB-ElOC pentameric complex 4nnj 
Crystal structure of Uba1 in complex with ubiquitin-AMP and thioesterified ubiquitin 4npn 
4NPN 4nqk 
Structure of an Ubiquitin complex 4nql 
4NQL 4p4h 
4P4H 4p5o 
4P5O 4pig 
4PIG 4pih 
4PIH 4pij 
4PIJ 4pqt 
4PQT 4q5e 
4Q5E 4q5h 
4Q5H 4r62 
4R62 4rf0 
4RF0 4rf1 
4RF1 4s1z 
4S1Z 4s22 
4S22 4uel 
4UEL 4uf6 
4UF6 4ug0 
4UG0 4ujc 
4UJC 4ujd 
4UJD 4uje 
4UJE 4un2 
4UN2 4v3k 
4V3K 4v3l 
4V3L 4v7e 
4V7E 4v88 
4V88 4v8m 
4V8M 4v8p 
4V8P 4v8t 
4V8T 4v8y 
4V8Y 4v8z 
4V8Z 4v91 
4V91 4w9c 
4W9C 4w9d 
4W9D 4w9e 
4W9E 4w9f 
4W9F 4w9g 
4W9G 4w9h 
4W9H 4w9i 
4W9I 4w9j 
4W9J 4w9k 
4W9K 4w9l 
4W9L 4whv 
4WHV 4wjn 
4WJN 4wjo 
4WJO 4wjp 
4WJP 4wjq 
4WJQ 4wlr 
4WLR 4wqo 
4WQO 4wur 
4WUR 4wzp 
4WZP 4xkh 
4XKH 4xkl 
4XKL 4xof 
4XOF 4xok 
4XOK 4xol 
4XOL 4xyz 
4XYZ 4y1h 
4Y1H 4z9s 
4Z9S 4zfr 
4ZFR 4zft 
4ZFT 4zpz 
4ZPZ 4zqs 
4ZQS 4zux 
4ZUX 4zyn 
4ZYN 5a5b 
5A5B 5aek 
5AEK 5af4 
5AF4 5af5 
5AF5 5af6 
5AF6 5ait 
5AIT 5aiu 
5AIU 5aj0 
5AJ0 5apn 
5APN 5apo 
5APO 5b83 
5B83 5bnb 
5BNB 5bo4 
5BO4 5bz0 
5BZ0 5c1z 
5C1Z 5c23 
5C23 5c7j 
5C7J 5c7m 
5C7M 5caw 
5CAW 5chf 
5CHF 5chv 
5CHV 5chw 
5CHW 5cra 
5CRA 5cvm 
5CVM 5cvn 
5CVN 5cvo 
5CVO 5d0k 
5D0K 5d0m 
5D0M 5d2m 
5D2M 5d6j 
5D6J 5dfl 
5DFL 5dk8 
5DK8 5e6j 
5E6J 5edv 
5EDV 5elj 
5ELJ 5elu 
5ELU 5emz 
5EMZ 5eql 
5EQL 5eya 
5EYA 5fer 
5FER 5flx 
5FLX 5gak 
5GAK 5gjq 
5GJQ 5go7 
5GO7 5go8 
5GO8 5gob 
5GOB 5goc 
5GOC 5god 
5GOD 5gog 
5GOG 5goh 
5GOH 5goi 
5GOI 5goj 
5GOJ 5gok 
5GOK 5hpk 
5HPK 5hpl 
5HPL 5hps 
5HPS 5hpt 
5HPT 5ibk 
5IBK 5ifr 
5IFR 5j8p 
5J8P 5jbv 
5JBV 5jby 
5JBY 5jg6 
5JG6 5jne 
5JNE 5jp1 
5JP1 5jp3 
5JP3 5jqs 
5JQS 5jtj 
5JTJ 5jtv 
5JTV 5juo 
5JUO 5jup 
5JUP 5jus 
5JUS 5jut 
5JUT 5juu 
5JUU 5jze 
5JZE 5k9p 
5K9P 5kgf 
5KGF 5khy 
5KHY 5l8h 
5L8H 5l8w 
5L8W 5l9t 
5L9T 5l9u 
5L9U 5lli 
5LLI 5ln1 
5LN1 5lrv 
5LRV 5lrw 
5LRW 5lrx 
5LRX 5lzs 
5LZS 5lzt 
5LZT 5lzu 
5LZU 5lzv 
5LZV 5lzw 
5LZW 5lzx 
5LZX 5lzy 
5LZY 5lzz 
5LZZ - Links (links to other resources describing this domain)
-
PROSITE UBQ_DOMAIN PFAM ubiquitin INTERPRO IPR000626

